How Much Water Can Air Hold?
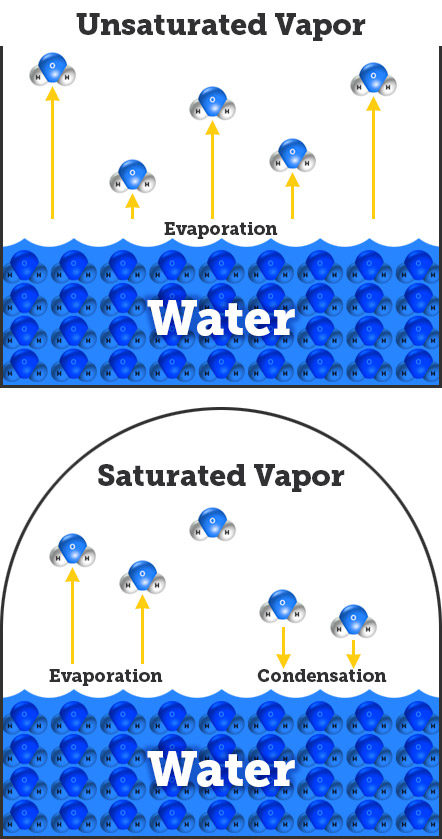
- When water evaporates, its energy allows it to escape the attraction to the other water molecules. But, another force is working on the water vapor molecules: pressure.
- Once a molecule escapes into the air, air pressure keeps it close to the surface, where it can lose energy and condense back into liquid water. The higher the air pressure, the harder it is for evaporation to occur.
- Water molecules are constantly evaporating and condensing. The pressure exerted by the water vapor molecules when evaporation and condensation occurs at equal rates is called the equilibrium vapor pressure, or saturated vapor pressure.
Source: WeatherSTEM